|
Inoculating and pelleting pasture legume seed
Greg Gemell, Technical Officer, Gosford Warren McDonald, Technical Specialist (Pastures), Tamworth.
Introduction
Is inoculation necessary?
Effective or ineffective nodulation?
Inoculants preparation and quality
Inoculants groups
Methods of inoculating seed
Lime pelleting
Adding molybdenum at inoculation
Re-inoculating seed
Pre-inoculated seed
When to sow
Environmental factors affecting survival of rhizobia
Compatibility of rhizobia with agrochemicals
Inoculation failure
Acknowledgments
Always Read The Label
Users of agricultural (or veterinary) chemical products must always read the label and any Permit, before using the product, and strictly comply with the directions on the label and the conditions of any Permit. Users are not absolved from compliance with the directions on the label or the conditions of the Permit by reason of any statement made or omitted to be made in this publication.
Pasture improvement may be associated with an increase in the incidence of certain livestock health disorders. Livestock and production losses from some disorders is possible. Management may need to be modified to minimise risk. Consult your veterinarian or adviser when planning pasture improvement.
The Native Vegetation Conservation Act 1997 may regulate some pasture improvement practices where existing pasture contains native species. Inquire through your office of the Department of Land and Water Conservation for further
details.
Disclaimer
The product trade names in this publication are supplied on the understanding that no preference between equivalent products is intended and that the inclusion of a product does not imply endorsement by NSW Agriculture over any other equivalent product from another manufacturer.
All legumes used in agriculture can use atmospheric nitrogen after it is ‘fixed’ inside nodules on their roots by a soil bacterium, Rhizobium. The rhizobia receive growth substances from the legume, and the legume receives nitrogen compounds for its growth from the rhizobia. That is, the rhizobia and the legume plant live together in a symbiotic or mutual benefit relationship.
A degree of specificity exists between types of legumes and strain of Rhizobium. For example, the strain which nodulates subterranean clover does not nodulate
lucerne.
Unless the roots of the legumes are well nodulated by effective strains of rhizobia, they will derive their nitrogen from the soil and thus deplete soil nitrogen. Companion grasses in a pasture benefit from the nitrogen fixed by the legume when it is recycled over time. The cereal yield and grain quality is also improved in a subsequent cropping phase.
When high numbers of the appropriate rhizobia are not present in the soil, they must be introduced by adding them to the seed at sowing, by a process called inoculation. Inoculated seed may also be pelleted by adding a protective layer of fine lime to improve survival of the rhizobia — particularly when sowing into acid soils, or if inoculated seed is in direct contact with acid fertilisers such as superphosphate or alternative products.
The rhizobia, and the nodules they form, are classified as ‘effective’ or ‘ineffective’ on the basis of how much nitrogen they convert (fix) to organic nitrogenous compounds (fixed nitrogen). When nodules are effective they fix most of the nitrogen the legume requires. Some of the nitrogen is returned to the soil via the grazing animal or by breakdown of legume organic matter. However, when pasture is cut for hay much of the fixed nitrogen is removed so that accumulation of nitrogen in the soil is considerably reduced.
The amount of nitrogen added to the soil from well-nodulated legumes varies greatly depending on the particular legume, the effectiveness of the bacteria, growing conditions and crop management. Soil nitrogen accumulation under subterranean clover pastures in Australia has been measured at between 39 and 81 kg N per hectare per year. Accumulation rates under lucerne in northern NSW may be as high as 140 kg N per hectare per year. The amount of nitrogen fixed under low-density legume pastures is often very low, and where weeds are numerous, soil nitrogen may decline rather than increase.
| Is inoculation necessary? |
Top |
If there is any doubt as to whether inoculation is necessary, then it is recommended that seed be inoculated. It is inexpensive insurance. When deciding whether to inoculate, consider the following:
* Is the legume new to that particular paddock?
* If not new, how many years has it been since well-nodulated plants of the same species or of a species from the same inoculant group were present?
* For how many years did they grow there?
* Is soil moisture, temperature and pH generally suitable for good survival of rhizobia? (See the section Environmental factors affecting survival of
rhizobia.)
Some
generalizations may be made
Always inoculate legume seed when growing in new areas and for legumes with specific rhizobia requirements, e.g. lotus, lotononis and Kenya clover.
Medics and most clover species are likely to be nodulated to some extent by local soil rhizobia. These rhizobia may not be effective in fixing nitrogen. Therefore, all species should be inoculated for new sowings and where a reasonable cover of well-nodulated plants of the same species (or the same inoculant group) has not been present for the last 2 years.
To compare legumes which have been nodulated by the rhizobia in the inoculant with those which have been nodulated by soil rhizobia, leave one short drill strip uninoculated at sowing. Plant growth and nodulation in the inoculated area can then be assessed with those in the uninoculated strip.
Figure 1. A good response to the use of inoculant. The dark green vigorous strips were sown with inoculated seed. The yellow to light green strips were from seed which was not inoculated.

The cost of inoculation and pelleting is relatively small and the procedure simple. Nodulation failure can be extremely expensive to overcome because resowing is the only solution for most dryland situations. Sometimes direct drilling or oversowing freshly inoculated legume seed into unsatisfactorily nodulated pasture may be necessary.
| Effective or ineffective nodulation? |
Top |
The nitrogen-fixing ability of nodules may be assessed by their appearance and by the colour and growth of the pasture. With newly sown pasture, allow 6 weeks before assessing nodulation. Nodules are best recovered by carefully digging a plant including a core of soil around its roots and gently washing away the soil to expose the roots. Extra care is needed with lucerne because it is very difficult to retain nodules unless the plants are young seedlings.
Check the nodules growing on the roots for colour, size, position and distribution. Slice some nodules to check their internal colour. Effective nodules are usually larger, are pink in colour and, if formed by the inoculum strain, tend to be found near the crown of the plant (see Figure 2). Ineffective nodules are small and white, are usually more numerous than effective ones, and are scattered over the root system. This occurs because the ineffective rhizobia are spread through the soil profile.
Figure 2. Effectively nodulated white clover plants. Note the large nodules are close to the crown of the plant.
In older nodulated plants some nodules may be green inside or green with pink tips. This indicates that the nodule is old or has aged due to stress. The green colour indicates the breakdown product of the pink leghaemoglobin, which is essential in the nitrogen fixation process.
Nodules with no pigment inside are ineffective, and those with only a small amount of pigment are only partially effective, in fixing nitrogen (see Figure 3). Ineffective nodulation in soils of low nitrogen status causes plants to be stunted and yellow, with the older leaves yellowing and dying first.
Figure 3. Nodules sliced in half. The ineffective nodules on the right-hand side are small and white. Pigmented pink nodules are effective, while the nodules on the far left are old and breaking down.
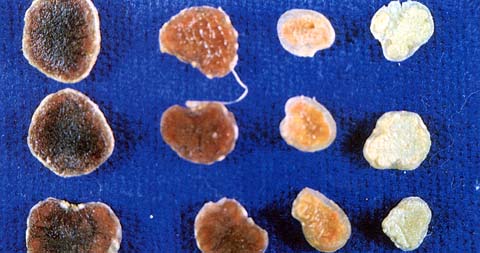
A simple test to verify ineffective nodulation is to thoroughly wet the leaves of legumes growing in a small area with a spray of 0.1% ammonium nitrate (Nitram®, i.e. 1.0 gram per litre of water) or urea. A marked positive growth response within a fortnight, if growing conditions are good, indicates that nitrogen is limiting growth. No response indicates that factors other than nitrogen are retarding growth.
| Inoculants preparation and quality |
Top |
Inoculants consist of finely ground peat soil impregnated with a single strain of rhizobia. Selected, highly effective, tested strains of rhizobia are first grown in nutrient broth which is then injected into packets containing sterilised peat, where the rhizobia multiply. Packets from each batch are tested by the Australian Legume Inoculants Research Unit (ALIRU), and only those batches which reach the stringent standards required are released for sale. Each packet has an expiry date.
The establishment of an effective strain of rhizobia depends on the number present on seed at sowing. The minimum number needed, however, is occasionally questioned. The recommendation (from the ALIRU) for rhizobia numbers on seed at sowing is 1000 viable rhizobia per seed of medium-sized seeds such as lucerne and subterranean clover, and 500 rhizobia per seed for small seeds such as white clover. These numbers will not guarantee adequate nodulation under all conditions.
In some cases other constraints, e.g. drought, highly acid soils, highly sodic soils etc., will suppress nodulation. However, the recommended standards do represent realistic and attainable numbers of rhizobia that should ensure adequate nodulation under most conditions of plant establishment and growth.
Among the rhizobia there is considerable variation. They differ in which legumes they can infect and how much nitrogen they supply to the plant. Common agricultural legumes have been classified into groups, each of which requires its own strain. Each group consists of related plants such as medics, but important exceptions include clovers which are divided into a number of groups. It is essential to purchase inoculant of the correct group.
| Methods of inoculating seed |
Top |
Pasture seed can be custom-inoculated by seed retailers, or can be inoculated on the farm just prior to sowing. The advantage of on-farm inoculation is that it can be done closer to sowing time, thereby reducing death of rhizobia before sowing. Death occurs during storage of inoculated seed.
The methods of inoculating seed are as follows.
Dry inoculation (not recommended)
This method involves adding peat inoculum to seed, and mixing. It is not recommended because attachment to the seed is poor and much of the inoculant is lost from the seed before and during sowing. The survival of the rhizobia which remain on the seed is also poorer than for other methods, as the rhizobia have little protection.
Slurry inoculation
This is a recommended method of inoculating grain legumes. It is also suitable for pasture seed that is to be sown into moist soil and not in direct contact with
fertiliser.
* Prepare a 0.5% methyl cellulose glue solution by dissolving 5 g methyl cellulose in 200
mL hot water (80°C). Then add 800 mL of cold water, and mix. Allow to cool.
* Mix 250 g of peat inoculant with the glue until evenly dispersed.
* Pour the mix over:
25 kg small seed (such as white clover, lucerne); or
50 kg medium seed (such as subterranean clover); or
100 kg large seed (such as lablab or centrosema).
* Mix until seeds are evenly coated. A thoroughly cleaned concrete mixer is suitable.
* Spread seed in a cool place out of direct sunlight until dry if required.
Inoculation
ingredients per kg of seed
|
Glue
(0.5% or 1.5% solution) |
Peat
inoculant |
Lime |
Small
seeds
(white clover and lucerne) |
40
mL |
10
g |
200
g |
Medium
seeds
(sub clover) |
20
mL |
5
g |
250
g |
Large
seeds
(lablab, centrosema) |
10
mL |
2.5
g |
125
g |
The main purpose of lime pelleting is to protect the rhizobia by counteracting mild soil or fertiliser acidity close to the seed. It also ensures better survival of the rhizobia when delays between pelleting and sowing are unavoidable.
Lime pelleting is not required when sowing tropical legumes, but may be of benefit in extremely acid soils, i.e. where pH < 4.0(Ca), or if sowing seed which is in contact with fertiliser. However, there are
exceptions
* Leucaena and
Kenya clover should be lime pelleted.
* Serradella, a temperate legume, should
not be lime pelleted.
* Biserrula, a recently introduced pasture legume, should
always be lime pelleted.
The method for lime pelleting seed is as follows:
1. Prepare a 1.5% methyl cellulose glue solution (15 g methyl cellulose to 1 L of water) — use hot water as described earlier in the slurry inoculation method. After cooling, mix 250 g peat inoculant with the glue until evenly dispersed.
2. Pour the mixture over the seed as for slurry inoculation and mix in a rotating drum (concrete mixer) until seeds are evenly coated.
3. Add the appropriate amount of very fine lime (such as Microfine® or Omyacarb® or plasterer’s whiting) in one step to the rotating seed, and roll for 1–3 minutes. Do not use builder’s lime (quicklime).
4. Allow pelleted seed to dry in a cool place out of direct sunlight.
This next step is optional
After pelleting but before drying, overspray the coated seed with a mist spray 1:1 solution of polyvinyl acetate (PVA woodwork glue) in water until the surface is damp. Continue rolling until the lime coat is firm.
Do not include this step if the seed/inoculant is not well coated with lime, as some PVA glues have preservatives that may be harmful to rhizobia.
Alternatively, a 1:1 mixture of the original methyl cellulose glue mix in water solution can be used; however, it is inferior to PVA glue. The PVA glue used in the optional final step binds the outside of the pellet which otherwise may be soft and powdery. This will result in pelleted seed flowing more freely when drilling.
Characteristics of a good pellet
* The seed should be evenly coated with the lime.
* Pellets should be firm enough when dry to withstand light rolling between the fingers.
Figure 4. Subterranean clover seed before and after pelleting. The lime coating should be even and firm.

Likely causes of poor pellets
Powdery, soft pellets indicate too much lime or uneven mixing, or both. Pasty-looking pellets with the seed surface showing is an indication of too much glue. This may be rectified by adding more lime. Clumps of small seed may also be caused by too much glue or inadequate mixing prior to adding lime. Hard, glossy, smooth pellets indicate too little lime, or too much mixing after adding the lime. The coating has a tendency to crack and flake off on drying, especially when handled.
A little experience in pelleting seed will help to overcome minor difficulties such as these. Adjustments in procedure may be needed for best pellet formation, depending on the machinery and technique used.
| Adding molybdenum at inoculation |
Top |
Molybdenum (Mo) may be added to seed directly at inoculation rather than applying it as a fertiliser additive at sowing. This is more cost-effective and ensures even distribution of molybdenum in the paddock.
Form of molybdenum
The usual fertiliser form of molybdenum is sodium molybdate, which is 39% pure molybdenum.
WARNING: Sodium molybdate is toxic to rhizobia and should not be used. Use either molybdenum
trioxide (66% Mo) or ammonium molybdate (54% Mo).
Rate of molybdenum required
When sowing pasture legumes in molybdenum-deficient soils, 50 g Mo is required per hectare, which is equal to that supplied in 250 kg/ha 0.02% Mo superphosphate. Then each 4-5 years, 25 g Mo should be applied per hectare as a maintenance dressing (e.g. 125 kg/ha of 0.02% Mo superphosphate). For example, if using molybdenum trioxide, containing 66% Mo, in the pelleting process, the total amount of product needed to supply 50 g Mo/ha is 76 g of molybdenum trioxide (MoO3). In some areas, responses to larger quantities of molybdenum have occurred. Check local recommendations. Do not apply higher rates of molybdenum than those recommended.
A batch of 50 kg of subterranean clover would require the addition of 375 g MoO3. Similarly, a 25 kg batch of lucerne seed would require 192 g MoO3. If sowing a mix of 4 kg/ha subterranean clover and 6 kg/ha lucerne, then 30 g MoO3 should be added for every 4 kg of subterranean clover that is inoculated, and 46 g MoO3 should be added for every 6 kg of lucerne seed that is inoculated, a total of 76 g MoO3 for every 10 kg of seed mix. Difficulties may be experienced applying sufficient molybdenum trioxide to the seed at low seeding rates, to provide the required rate of molybdenum per hectare. If this occurs the balance should be added mixed with fertiliser in the traditional way.
The quality of molybdenum trioxide can vary, so a check should be made when determining the rate to use.
Method
* Increase the amount of glue solution by 5% to allow for the extra dry material.
* Mix the inoculant and glue, pour onto seed and mix until seed is evenly moistened.
* Add the appropriate amount of molybdenum trioxide to the seed proportionally by weight, and mix until evenly coated.
* Add the normal amount of lime and mix until evenly
pelleted.
Where inoculated and pelleted seed (as above) has not been sown within 1 week of seed pelleting, it is recommended that the treated seed be re-inoculated. Two methods may be used:
* Dry inoculate and sow
immediately That is, mix peat inoculant with the pelleted seed in the same proportions as before. Some peat will adhere to the outside of the old lime coating. The rhizobia will not have the same protection as under the pellet; however, they should receive some benefit from the lime depending on the pH of the soil.
* Re-inoculate and
pellet This is the more desirable method where a lime pellet is seen as essential. This can be carried out normally with the glue/inoculant layer going over the old pellet, then adding the lime as normal, allowing for the extra bulk when estimating quantities. One problem is that the old pellets can fall apart during the operation. This may be minimised by binding the old pellet first by spraying a very light mist of a 1:1 mixture of methyl cellulose in water. Keep the mix rotating while spraying and drying.
Pre-inoculated seed is available using specialised coating processes. This can extend the potential for survival of rhizobia beyond the accepted 7 days for traditional lime pelleting. This has potentially provided more flexibility to handle problems such as sowing delays. It is advisable, however, to sow as soon as possible after seed treatment.
Processes vary, as does the quality of the product. Recent testing of inoculated seed samples collected from retail outlets indicated that many samples did not have sufficient numbers of rhizobia present per seed for optimal nodulation.
Unfortunately there is no rapid test to assess adequacy of numbers of rhizobia on the seed. If there is any doubt about the survival of rhizobia or the shelf life of a pre-inoculated seed product, contact the supplying company regarding the need to re-inoculate.
Inoculated seed must be kept out of sunlight and as cool as possible, preferably around 15°C, until ready to sow. Slurry-inoculated seed must be sown within 12 hours of inoculation. Lime-pelleted seed should be sown within 24 hours, but if sowing is unavoidably delayed, store for up to 1 week at 15°C or less. However, aim to sow inoculated seed into moist soil immediately after inoculation in order to minimise death of rhizobia.
Do not sow inoculated seed in contact with any fertiliser except lime, dolomite or gypsum. If contact cannot be avoided, lime pellet the seed first and do not store it mixed in with the fertiliser — sow immediately.
When sowing lime-pelleted seed, the added lime will increase the bulk of the sowing mixture and also the weight. Adjust the combine to sow the correct amount of seed. The increase in weight and size can be significant and will depend on the seed size and the efficiency of pelleting. For example, subterranean clover seed may increase in weight by 25%, white clover by 50% and lucerne by 30%.
| Environmental factors affecting survival of rhizobia |
Top |
Temperature and moisture
Rhizobia numbers on seed are highest at the time of inoculation and decline subsequently. The decline is dependent on several factors, including:
* species/strain differences
* quality of glue/pelleting materials
* storage conditions
* time to sowing
* environmental conditions
High temperatures kill rhizobia. Rhizobia on seed are more sensitive to heat than when in unopened packets of peat inoculum. It is important that packets of inoculum are not subjected to high temperatures for long periods between leaving the manufacturer and their use on the farm.
Nearly all inoculants are best stored in a refrigerator. The exceptions are the inoculants for fine stem stylo, centrosema, desmodium and lablab, which should be stored between 20°C and 30°C.
Rhizobia in the soil are affected by both soil temperature and moisture. In hot dry soils, rhizobia mortality is greater near the soil surface. The surviving rhizobia at depth produce nodules further down the roots. The deeper the inoculated seed can be sown, while not affecting seedling establishment, the better the chances are of rhizobia survival.
Rhizobia on surface-sown seed and seed drilled shallow into the soil have an even poorer chance of survival and depend on early rain to wash and distribute them into the soil profile. Alternatively, the inoculant can be sprayed below the seed as is done for some legume crops.
Acid soil factors
Acid soil factors which adversely affect plants and rhizobia are:
* low pH
* low calcium (Ca) and phosphorus (P)
* high levels of aluminium (Al) or manganese (Mn).
A measure of soil pH is an initial indicator of potential adverse effects on plants and nitrogen fixation. As soil pH falls, calcium, phosphorus and molybdenum become less available for plant growth, nodulation and nitrogen fixation. If in doubt about soil acidity in your situation, consult your local District Agronomist for advice.
In general, rhizobia are more tolerant of these factors than are the plants. When sowing inoculated seed into acid soils, the seed should be lime pelleted. (See ‘exceptions’ in the section ‘Lime pelleting’). If the soil pH(Ca) is below 5.0, it is necessary to also sow with additional agricultural lime. The amount of lime will vary according to the soil pH and the method, such as whether the seed is broadcast or drilled. Your local District Agronomist will be able to advise on the precise rates for the particular situation.
Nitrogen fixation is very dependent on phosphorus and molybdenum. In deficient soils, phosphorus should be applied at sowing, while molybdenum may be applied with the superphosphate or incorporated into the lime pellet.
| Compatibility of rhizobia with agrochemicals |
Top |
Rhizobia are sensitive organisms, and many fertilisers, fungicides, insecticides and herbicides can harm them. Other chemicals may do only slight harm or have no effect. There are three major factors to be considered when attempting to predict any unfavourable interactions:
1. Are the chemicals acid in solution? Most rhizobia are sensitive to solutions of pH values below 5.0 and above 8.0.
2. Do the preparations contain toxic chemicals? Metals such as mercury, copper and zinc are harmful; the effect of other active ingredients may be difficult to predict.
3. Is there prolonged direct contact between the substance and inoculated seed? If direct contact is avoided, or is only brief, the effect may be
minimised.
Specific examples of interactions with inoculated seed follow.
Fertilisers
* Superphosphate and related products, because they are very acid, are toxic to rhizobia if in direct contact. Either the chemical’s acidity must be reduced by mixing with agricultural lime, or the seed must be lime pelleted. Even if lime pelleted, the time the seed is in contact with superphosphate should be as short as possible.
* Gypsum can be mixed with inoculated seed.
* Starter fertilisers used at low rates for pasture establishment, such as 45 kg/ha monoammonium phosphate, can be sown with lime-pelleted seed (no more than 20 kg N/ha with seed).
* Molybdenum as used for seed treatment can be toxic if the wrong form of molybdenum is used. Molybdenum trioxide is compatible; however, sodium molybdate is toxic to rhizobia.
Fungicides
Recent tests have shown that Metalaxyl and Metalaxyl-M, when applied to inoculated seed, contribute to a reduction in the number of rhizobia, and therefore should be used strictly in accordance with the manufacturer’s instructions.
Insecticides
* Bendiocarb and permethrin, used to protect seed from ants, are safe (although limited trials indicate that there may be some reduction in nodulation).
* Dimethoate can harm rhizobia.
* Imidaclophrid is safe to rhizobia provided treated seed is sown into moist soil within 1 day of treatment for subterranean clover and murex medic, and within 6 days of treatment for other species (i.e. white clover, serradella, lucerne and barrel medic). Follow label instructions carefully.
Herbicides
Rhizobia are relatively tolerant to herbicides at concentrations recommended for field use. Because of the differences in susceptibility between the host and their rhizobia, it is difficult to make accurate assessments.
When the toxicity of a particular herbicide is unknown, keep it separated from inoculated seed.
Where inoculation has failed, keep in mind the environmental and agrochemical compatibility factors outlined above when looking for the reason.
Attempts at introducing fresh inoculum into a failed paddock have had varied success, because high numbers of rhizobia are needed close to the seed and it is difficult to deliver the required number in an economical and practical way.
Usually the best method is to resow the pasture legume component of the mixture with freshly inoculated and pelleted seed. This may involve waiting until the following year for the optimum sowing time. When perennials are involved, greater flexibility exists. Low quantities of nitrogenous fertiliser (e.g. less than 20 kg N/ha) may assist in maintaining the health and vigour of the plant until nodulation occurs.
There are two experimental post-sowing methods:
* applying inoculant in irrigation water, known as ‘water run’ inoculation;
* applying inoculant through a boom spray (at least 100 L of suspended inoculum per hectare is needed).
These are experimental methods that have shown promise and are worthy of trial if resowing is out of the question. Both methods require very high rates of inoculum and that the inoculum must be washed down into the soil profile quickly for effective nodulation.
If peat inoculum is used in pumping systems, filtering of peat inoculum may be necessary to avoid blocking jets. Pressures greater than 175 kPa may kill rhizobia. Use potable water. Water with temperatures greater than 30°C may kill rhizobia.
Consult your District Agronomist or adviser for further advice.
The authors thank the following people for their technical assistance:
Dr D Herridge, NSW Agriculture, Tamworth
Mr R Gault, CSIRO, Canberra
Dr J Evans, NSW Agriculture, Wagga Wagga
Dr R Date, CSIRO, Brisbane, Queensland
J Slattery, Department of Natural Resources and Environment, Rutherglen, Victoria.
http://www.agric.nsw.gov.au/
|
Pakissan.com;
|