All About/Crops/Mashroom
Gene Transfer Technology for Mushrooms: The Power and Potential for Significant Crop Improvement
C. Peter Romaine
Department of Plant Pathology
The Pennsylvania State University
University Park, PA 16802
Birth of Recombinant DNA Technology
In the early 1970's, California scientists first succeeded at splicing viral and bacterial DNAs in the test tube, heralding the birth of the recombinant DNA (rDNA) era, popularly known as genetic engineering, gene transfer technology, gene splicing, molecular biotechnology, and transgenics. This new biotechnology found immediate application in the production of pharmaceuticals, where synthesis by rDNA microbes provided a quantum leap in efficiency over the laborious extraction of miniscule amounts from other sources. Early on it was stated that "the uses of biotechnology are only limited by the human imagination." Today we are witnessing how this broad-based science is impacting virtually every sector of our society.
It was during the 1980's when the power and potential of the burgeoning discipline of genetic engineering was first brought to bear on the improvement of agricultural productivity. The discovery of techniques to transfer genes to the major agronomic crops, including corn, soybean, and wheat, from unrelated species provided breeders with new vistas for increasing the efficiency of food crop production. Remarkable progress, far exceeding early predictions, has been made during the last two decades in breeding plants with new traits such as insect, viral, and fungal resistance, herbicide, stress, and cold tolerance, delayed senescence, improved nutritional features, and others. The global demand for transgenic crops is projected to be a $25 billion market by the year 2010. The growth of this industry will be propelled, in part, by "Golden" rice, which was engineered using a daffodil gene to be rich in beta carotene and thereby the promising answer to the vitamin A deficiency problem pervading the developing world.
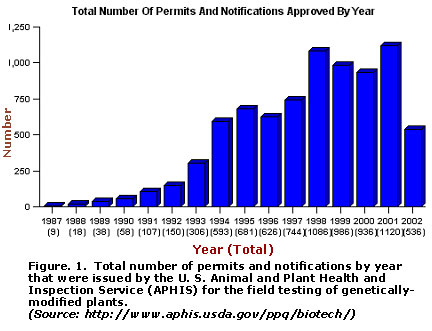
Despite concern for the unforeseeable health and environmental risks posed by genetically-modified (GM) crops, gene transfer technology has irreversibly revolutionized plant breeding. Today, more than 100 plant species have been modified by gene splicing for improved sources of food, fiber, or ornamentation. More than 50 new crop varieties have cleared all federal regulatory requirements and stand approved for commercial retail. Because field testing is an essential step in the commercialization process, the number of permits issued by the U. S. Department of Agriculture, Animal and Plant Health and Inspection Service (APHIS) for GM crops provides a measure of the interest in transgenic breeding. During a 16-year period, more than 8,000 permits and notifications (fast-track permits) were issued, rising from a low of 9 in 1987 to a high of 1,120 in 2001 (Fig. 1). For the first three months of 2002, 536 permits/notifications were recorded by APHIS with 49% involving insect resistance, 33% herbicide tolerance, 7% each for product quality and agronomic properties, and with the balance comprising fungal and viral resistance and other traits. Thus, the "genie out of the bottle" scenario describes the status of agricultural genetic engineering. Despite the anti-GM sentiment expressed by a vocal minority, the potency of the new biotechnology for problem solving has been realized to an extent that is far too compelling for it to be disregarded.
Genetically Engineering the Button Mushroom
For almost as long as scientists have been introducing genes into crop plants using molecular biotechnology, others have attempted with limited success at developing a gene transfer method for Agaricus bisporus. A major breakthrough came in 1995 with the surprising discovery that the bacterial workhorse, Agrobacterium tumefaciens, used to shuttle genes into plants, also operated with yeast fungi. Shortly thereafter, this method was extended to filamentous fungi, including A. bisporus.
Agrobacterium is a common soil bacterium with a worldwide distribution. It causes a disease known as crown gall on hundreds of woody and herbaceous plant species, but most commonly pome and stone fruits, brambles, and grapes. In its normal life cycle, the bacterium transfers a tiny bit of its DNA into the plant DNA resulting in the formation of galls. These galls serve as food factories for the mass production of the bacterium. Over the years, scientists learned how to develop disarmed strains of the bacterium that were incapable of inducing galls, but retained the ability to transfer DNA. In essence, a natural biological process was harnessed to create a bacterial delivery system for moving genes into plants, and now fungi.
Though Agrobacterium was shown to be highly promiscuous in shuttling genes into a spectrum of plant and fungal species, the method was still too inefficient to be applied to the breeding of A. bisporus. More recently, we devised a convenient and effective Agrobacterium-mediated 'fruiting body' gene transfer method holding the promise of a powerful tool for the genetic improvement of the mushroom. In our experiments, a small ring of DNA carrying a gene for resistance to the antibiotic, hygromycin, was transferred to a disarmed strain of the Agrobacterium. The antibiotic resistance gene is referred to as a selectable marker, because mushroom cells receiving this gene from the bacterium become marked by the resistance trait and can be selected based on the ability to grow on a hygromycin-amended medium. The end result is a mushroom strain having the newly acquired characteristic of hygromycin resistance. Such a strain has little commercial value, but rather the resistance trait was a research tool that allowed us to easily determine if the bacterium had transferred the gene to the mushroom, and exactly how efficiently it did so under different experimental conditions. Today, and more so in the future, this gene is being replaced or complemented by genes that will confer commercially relevant traits.
Figure 2 highlights the steps in the 'fruiting body' gene transfer method. In this procedure, gill tissue is taken from mushrooms approaching maturity, but with the veil intact, so as to ensure some degree of sterility. Next, the tissue is cut into small pieces and vacuum-infiltrated with a suspension of Agrobacterium carrying the antibiotic resistance gene. In a process referred to as co-cultivation, the gill tissue and bacterium are grown together in the laboratory for several days, during which time the bacterium transfers the resistance gene to the mushroom DNA. Because not all mushroom cells receive a copy of the gene, those that have can be distinguished from those that have not by the ability to grow on the antibiotic medium. After 7 days on the medium, mycelium of A. bisporus appears growing at the edges of some of the gill tissue pieces. After 28 days, upwards of 95% of the tissue pieces will have regenerated into visible cultures. At this point, the GM cultures can be transferred to a standard growth medium, and used to prepare grain spawn in the ordinary manner.
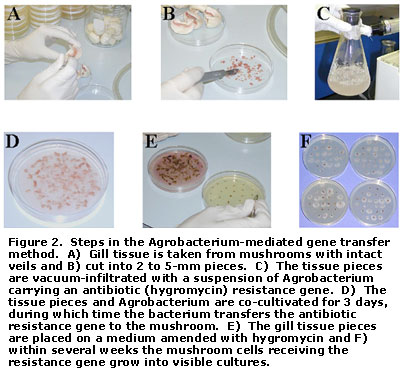
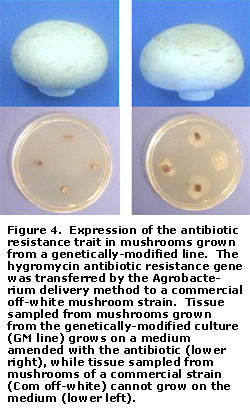
Figure 3 depicts the first of two cropping trials carried out at the Penn State Mushroom Research Center involving GM mushroom lines. In these trials, all six antibiotic-resistant GM lines mirrored the parental commercial hybrid strain in colonizing the compost and casing layer. Further, the GM lines produced mushrooms having a normal appearance and, in some cases, yielded on a par with the commercial strain (Table 1). Expression of the resistance trait in the mushrooms could be easily demonstrated by placing pieces of the cap or stem tissue on the antibiotic medium and observing for growth (Figure 4). These experiments were crucial, because the results established for the first time that a foreign gene could be introduced into A. bisporus without having a detrimental effect on its vegetative and reproductive characteristics.
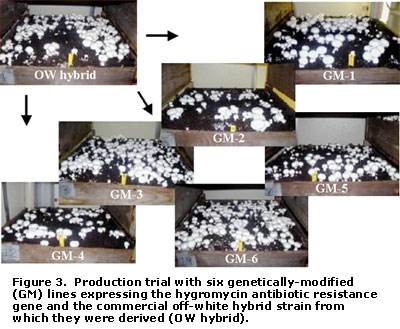
Table 1. Productivity of genetically-modified (GM) mushroom lines expressing the antibiotic resistance gene that were derived from a commercial off-white hybrid strain.
Yield (lbs./sq. ft.)
| Yield
(lbs./sq. ft.)
|
| Line |
Trial
I |
Trial
II |
| Commercial
hybrid |
3.00
a |
3.68
a |
| GM-1 |
2.08
d |
0.86
d |
| GM-2 |
1.73
d |
1.45
d |
| GM-3 |
2.52
bc |
2.70
c |
| GM-4 |
2.12
cd |
2.99
bc |
| GM-5 |
2.90
a |
3.63
a |
| GM-6 |
2.86
ab |
3.59
a |
Means within a column having the same letter are not significantly different according to the Waller-Duncan K-ratio t test at P<0.0001
Impact of Transgenic Breeding on Mushroom Cultivation
The overwhelming popularity of the hybrid mushroom strains introduced in the 1980's has created a near global monoculture that is precarious from the standpoint of disease and pest susceptibility, and has limited the choice of production characteristics and the range of tolerance to environmental and cultural stresses. During the last two decades, no notable advances have been made in breeding strains with strikingly improved features. This is due largely to the cumbersome genetics of A. bisporus and a shortage of commercially desirable traits. There is movement afoot in using traditional breeding to explore wild isolates of A. bisporus as a source of new traits. Though this represents an important step towards expanding the genetic base of cultivated A. bisporus, it is not yet clear which traits exist in the wild germplasm collection, and if they can be successfully bred into commercial strains.
The advent of a facile gene transfer technique for A. bisporus enables the exploration of genetic solutions to problems confronting the mushroom industry in a realm never before imagined. The awesome power of transgenics lies in what is known as the universality of the genetic code. The biochemical alphabet consisting of the letters G, A, T, and C that spells the DNA sequences of genes controlling traits is identical for all organisms. A scientist blindly handed a gene would have difficulty determining if its source was a mushroom, mouse, or man. It is this unifying feature of genes from all walks of life that makes transgenics so potentially powerful, while it is the tools of molecular biology that unleashes this power so this potential can be realized. Simply stated, the new biotechnology permits the exchange of genetic information between organisms outside the confines of the natural breeding barrier. No longer is the genetic improvement of the mushroom decided by the question of sexual compatibility or traits found within the species.
At another level, gene transfer technology will vastly accelerate our understanding of the molecular mechanisms underlying commercially relevant characteristics. It also will serve to strengthen the muscle of our industry's scientific arm, growing from a handful of mushroom researchers to the global workforce of molecular biologists. As one hypothetical illustration, the quest to breed robust resistance to dry bubble disease would not be restricted to a few scientists searching within A. bisporus, where it may or not exist. Instead, it would extend to scores of scientists working on unrelated organisms who have discovered resistance genes to other Verticillium species. Importing these genes to the mushroom for an evaluation against dry bubble is now possible. As farfetched as this may seem, it is precisely this trans-species approach that has met with commercial success. Genetic manipulations of this sort have been carried out on crop plants and include, importing cry genes from the Bacillus thuringiensis bacterium for insect resistance, a synthetase gene from Agrobacterium for glyphosate herbicide resistance, the nitrilase gene from the Klebsiella pneumoniae bacterium for bromoxymil herbicide resistance, a hydrolase gene from the Escherichia coli bacterium for modified fruit ripening, the barnase gene from Bacillus spp. for male sterility, and viral genes for virus disease resistance.
It cannot be overstated that gene transfer technology is not a panacea whose arrival marks the departure of traditional breeding. Quite the contrary, it is a new tool at the disposal of the breeder that will complement existing techniques, while offering a far broader range of options for successfully affecting genetic solutions to problems. Gene splicing will expedite the breeding process, transferring much of the time in development from the field to the laboratory. It will enable the introduction of genes with a surgical precision and from exotic sources, which otherwise would be unattainable by more conventional methods. It is important to recognize, however, that in the end, the forces of nature overcoming a trait (e.g., the breakdown of insect resistance) would act with the same intensity on the controlling gene whether introduced by traditional or transgenic breeding.
The melding of gene transfer methods with traditional techniques in a mushroom breeding program may take several forms initially, only to be continually refined, streamlined, and improved for higher efficiency and greater effectiveness. Many transgenic manipulations with A. bisporus will require the transfer of the gene to both parental lines so that their offspring mimic the natural inheritance process by carrying a duplicate copy of the gene. For other applications, introducing a single copy of the gene may achieve the desired effect. In either case, the resulting GM lines may require further selection before emerging as worthy commercial strains.
The Perils of Genetic Engineering
If the decision to exploit genetic engineering for agricultural improvement was left to scientists, the cultivation of GM crops would probably be far more widespread and diverse than it is today. But science does not occur in a vacuum. Political forces reflecting the pendulum of public opinion have a strong bearing on the direction and timetable of scientific progress. The early comment that, "the uses of biotechnology are only limited by the human imagination" was used within the context of its seemingly boundless benefit to humanity. In actuality, human imagination has limited biotechnology. That food crops created by genetic engineering are unnatural to the extreme of threatening human health and the delicate balance of the environment is a perception held by a segment of our society. Whether or not these fears are rationale is irrelevant, because their mere existence has hampered the growth of genetic engineering in agriculture. As with many new technologies, the question of acceptance by society will be answered through a distillation of the benefits to be derived for the risks that must be taken.
Both transgenic and conventional plant breeding strive to increase yield, improve quality, and reduce production cost. However, the two breeding strategies differ enormously in the manner in which the end is achieved. For many, it is the process of genetic engineering and not the final product that is most disconcerting. Removing the element of compatible sexual crosses from breeding and reducing it to the splicing of genes in the laboratory seems highly unnatural, constituting extreme human intervention. True, only transgenic breeding allows genes from exotic sources to be brought together in unique combinations. This has been criticized for the possibility of creating new and unpredictable food-borne allergies and toxicities. But the conclusions drawn by the American Medical Association, Board on Agriculture and Natural Resources and National Research Council, and Institute of Food Technologists, among other organizations, agree that GM food poses no greater threat to human health than conventional food.
Consider the tomato breeder seeking to transfer disease resistance from a wild species to the cultivated species. The traditional approach would be to cross the wild species with the domesticated species producing an offspring having inherited half of its genes from one parent and half from the other. In an effort to filter out the undesirable traits contributed by the wild species, the breeder would repeatedly cross the offspring with the cultivated species. However, this process is imperfect, so the new commercial tomato variety would possess the resistance gene and other contaminating genes from the wild species. Now, in the transgenic approach, the resistance gene alone would be snipped from the DNA of the wild species and transferred to the cultivated variety. Here, the risk of altering the food constituents is greater with the conventionally bred variety than the GM variety. For this reason, a likely backlash of the genetic engineering controversy will be stricter regulation of food crops bred by conventional means. As a matter of fact, GM food is federally regulated and adequate safeguards for quality assurance are in place. There is no logical reason to believe that a GM food product would be any more threatening to human health or any more difficult to evaluate for safety than say, for example, a new drug.
Another safety concern with GM food crops revolves around the unintended consequences associated with introduced genes escaping GM crop plants to other species. Science cannot predict with absolute certainty the non-targeted effects of GM crops on the environment, but it can determine which native species could acquire an escaped gene by cross-pollination. More importantly, science is now beginning to appreciate that genetic exchange among unrelated organisms occurs in nature. Therefore, it can be argued that moving genes between unrelated species by transgenic breeding only accelerates this natural evolutionary process.
In order for GM food to reach mainstream society, a greater emphasis must be placed on trait improvements that will benefit the consumer. Most of the genetic engineering accomplishments with crop plants have involved input traits, such as herbicide tolerance and disease and insect resistance. Farmers have embraced the new biotechnology with open arms, because GM crops have reduced their workload or increased profit. But what incentives exist for the consumer to choose GM over non-GM produce in the marketplace? The Agbiotech giants now realize this and are redirecting research towards output traits offering greater consumer appeal, as for example, improved shelf life, appearance, color, flavor, nutrition, hypoallergenicity, etc.
The Shape of the Future
The pace at which genetic engineering is implemented in the mushroom industry will be determined solely by economic factors related to necessity and the resources committed to R & D. If transgenic breeding offered a solution to a problem threatening the livelihood of the mushroom industry today, then the growing of GM strains would become widespread tomorrow. This 'do or die' scenario played out in Hawaii, where a ringspot virus was literally decimating the papaya industry. Fortunately, the fruits of a transgenic breeding effort underway for many years provided a solution. Virtually all papayas now produced in Hawaii are GM for virus resistance.
Another economic force driving the rate at which transgenic breeding reaches the mushroom industry is the level of emphasis placed on R & D. Mushrooms lag far behind other crops in molecular biotechnology, so it is likely that the path through public opinion to acceptance of GM food will be forged by these other commodity groups. As the climate for GM food improves, so will the research funding for the transgenic breeding of mushrooms. For the time being, scientific meetings will be punctuated by modest advances in mushroom transgenics contributed primarily by laboratories in Europe and Far East.
Early transgenic breeding achievements with mushrooms will likely shadow those on cultivated crop plants. Because of funding constraints and technical ease, traits controlled by single genes will be targeted initially, including viral and fly resistance and possibly resistance to bacterial and fungal pathogens, pesticides, and bruising. With the mapping of the mushroom genome and an increased understanding of genetic mechanisms, complex traits controlled by more than a single gene will be undertaken. Improvements might be expected in the areas of yield, size, color, shelf life, heat and water stress, food constituents, fruiting cycle regulation, sexual compatibility, strain stability, and substrate utilization.
Mushrooms will be explored as bioreactors for the synthesis of valuable pharmaceuticals and other bioproducts. The idea of growing the mushroom as a factory rather than a food offers several possible advantages over existing plant-based schemes (i. e., tobacco and corn). A high biomass of mushrooms can be produced on low-cost waste material in a secure containment facility with a controlled, HEPA-filtered environment, and with the option for mechanical harvesting. Further, it may be learned that proteins manufactured by mushrooms have higher specific biological activities in humans than those produced in plant counterparts.
By virtue of the foreign gene introduced and its location within the mushroom DNA or, alternatively, through the deliberate introduction of small snippets of DNA as molecular signatures, it will be possible for spawn manufacturers to definitively identify their strains. This ability to fingerprint strains with ease will afford greater patent protection, which, in turn, will provide the resources to expand breeding programs. The economic incentives related to patented strains also may attract new, perhaps venture capital funded parties to strain development and spawn manufacturing. The mushroom industry as a whole would benefit from the increased competition through a greater selection and diversity of mushroom strains.
Our industry is on the brink of a new and exciting age of strain improvement of a like never experienced before. Many of the accomplishments being realized for cultivated crop plants through transgenic breeding might now be achieved for mushrooms. The availability of mushroom strains with genuinely novel and obviously improved traits will provide the industry with new options for solving problems, simplifying tasks, increasing the efficiency of production, and usage. Though the timetable for its application to mushroom cultivation remains an uncertainty, to paraphrase, "genetic engineering will be persistent, it will be pervasive, and it will be everlasting."
Source: http://biotech.cas.psu.edu/
|
Other Global trends
|
DISCUSS
issue
problems
at
Pakissan Forum
Connect with
the
Pak Agri Community
|
Register
Today at
Pak
APIN
(Pakissan
Agri Experts and Institutes Network)
& become
part of the
Agri
Community
of
Pakistan
|
|
|