|
A tree with a purpose Gliricidia sepium
A tree with several useful properties and incredible potential has been
introduced to Pakistan
The Pakistan Agriculture Research Council has recently acquired a leguminous
tree with amazing potentials and several properties that can kill field rats, a
serious enemy of agriculture. The rats die due to haemorrhage in the guts, lungs
and spleen, just like the costly, hard to obtain coumerin does without harming
cattle and human beings.
 This makes it an ideal agriculture-friendly flora unlike the repugnant,
repulsive, belligerent and invasive prosopis that entered the subcontinent
gently to fight the slow yet dangerous advance of the desert, but later which is
about fifty years from today, it took a smothering turn to grow profusely under
the harshest conditions, including the persistent drought of Sindh, Balochistan
and much of southern Punjab, usurping land that it was supposed to protect. This makes it an ideal agriculture-friendly flora unlike the repugnant,
repulsive, belligerent and invasive prosopis that entered the subcontinent
gently to fight the slow yet dangerous advance of the desert, but later which is
about fifty years from today, it took a smothering turn to grow profusely under
the harshest conditions, including the persistent drought of Sindh, Balochistan
and much of southern Punjab, usurping land that it was supposed to protect.
Technically known as Gliricidia sepium from the Fabaceae family, which literally
means "rat poison", it is indigenous to Central America, where, on account of
its benevolent nature, it is known as Madre de Caco (mother of cocoa), a useful
tree that will be of immense help in increasing the yield of crops in Pakistan
where the soil is, more so in the South of the country desperately in need of
regeneration.
Introduction
Gliricidia sepium is a medium-sized
leguminous tree which occurs in abundance throughout its native range in
Mesoamerica. Domestication of gliricidia has been in progress for several
millennia and the multitude of indigenous common names from Mayan and Quiche
peoples (Pertchik and Pertchik 1951) reveals the importance of this species to
early occupants of the region. Spanish colonists adapted the local vernacular in
naming the species 'madre de cacao' (mother of cocoa) to describe its use
as a cocoa shade tree. The toxic properties of the seeds and bark of G.
sepium give rise to the generic epithet of this species (Gliricidia =
mouse killer) as well as a number of common names (e.g. mata-raton). Present day
uses of this species throughout the native range (e.g. firewood, living fences,
shade, construction and as an ornamental) are likely extensions of early
utilization and popularity (Rico-Gray et al. 1991).
 Gliricidia sepium has also been used
extensively outside its native range in places which include the Caribbean, the
Philippines, India, Sri Lanka and West Africa. These landrace populations are
largely remnants of colonial introductions used to shade plantation crops
although more recently they have been integrated into indigenous farming
practices being used for fuel wood, living fences, animal forage, green manure
and soil stabilization. Gliricidia sepium has also been used
extensively outside its native range in places which include the Caribbean, the
Philippines, India, Sri Lanka and West Africa. These landrace populations are
largely remnants of colonial introductions used to shade plantation crops
although more recently they have been integrated into indigenous farming
practices being used for fuel wood, living fences, animal forage, green manure
and soil stabilization.
After Leucaena leucocephala, G. sepium is
believed to be the most widely cultivated multipurpose tree. In many cases,
gliricidia will yield as much as or more biomass than L. leucocephala
(Stewart et al. 1992). One of the reasons for its recent popularity is
its complete resistance to the defoliating psyllid (Heteropsylla cubana)
which has devastated L. leucocephala in many parts of the tropics.
This section describes the taxonomy, ecology, distribution and general uses of
G. sepium, as a prelude to discussion of its use as a forage species.
Botanical description
Gliricidia sepium is a small to medium-sized, thornless tree which
usually attains a height of 10-12 m. Branching is frequently from the base with
basal diameters reaching 50-70 cm. The bark is smooth but can vary in colour
from whitish grey to deep red-brown. The stem and branches are commonly flecked
with small white lenticels. Trees display spreading crowns. Leaves are odd
pinnate, usually alternate, subopposite or opposite, to approximately 30 cm
long; leaflets 5-20, ovate or elliptic, 2-7 cm long, 1-3 cm wide. Leaflet midrib
and rachis are occasionally striped red. Infloresences appear as clustered
racemes on distal parts on new and old wood, 5-15 cm long, flowers borne singly
with 20-40 per raceme. Flowers bright pink to lilac, tinged with white, usually
with a diffuse pale yellow spot at the base of the standard petal, calyx
glabrous, green, often tinged red. Standard petal round and nearly erect,
approximately 20 mm long; keel petals 1520 mm long, 4-7 mm wide. Fruit green
sometimes tinged reddish-purple when unripe, light yellow-brown when mature,
narrow, 10-18 cm long, 2 cm wide, valves twisting in dehiscence; seeds 4-10,
yellow-brown to brown, nearly round (modified from C.E. Hughes, unpublished
data) (Figure 2.2.1).
Systematics
Gliricidia is a member of the sub-family Papilionoideae and lies within
the tribe Robinieae (Lavin 1987). The genus Gliricidia, which has been
previously ascribed to Lonchocarpus and Robinia, comprises a
small, yet debated, number of taxa. It is most commonly known by its
pink-flowered species, G. sepium, which is routinely observed throughout
its natural range in the dry forest of the Pacific coast of Central America and
Mexico (Hughes 1987). A closely related white flowered taxon, G. maculata,
is less common although it is frequently confused with G. sepium despite
its disjunct distribution in the Yucatan Peninsula. Most confusion of these two
taxa has arisen in exotic locations where they are often treated as synonyms
(see for example Falvey 1982) thus resulting in indiscriminate use of
nomenclature in forestry literature (Whiteman et al. 1986, Reynolds 1988,
Joseph et al. 1991).
Despite the sexual compatibility of these two
taxa (A.J. Simons, unpublished data), there exists substantial evidence to
confirm Rydberg's (1924) treatment of the white flowered entity as a distinct
species. Lavin et al. (1991) showed distinction between G. sepium
and G. maculata based on studies of chloroplast DNA polymorphisms. Simons
and Dunsdon (1992) present 12 separate characters that can be used to
distinguish these species, some of which have only recently become known (e.g.
molecular markers, seed diameter, stem form) with the provision of trial
material grown under uniform conditions.
Fig. 1. Leaves, flowers and pod
of Gliricidia sepium.
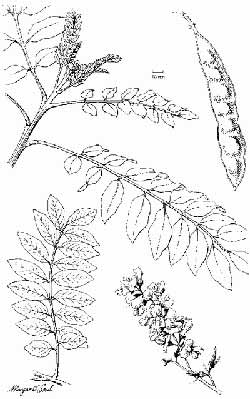
Ecology
Despite the widespread present occurrence of
G. sepium in cultivation throughout Central American countries and Mexico,
it is likely to be native only in the seasonally dry forest (Hughes 1987). It is
largely deciduous during the dry season which runs from January to the first rains in May. In areas where sufficient
moisture prevails, however, the tree does not become leafless (e.g. Kalimantan,
Indonesia; Seibert 1987). Flowering begins at the start of the dry season and
can continue in some native populations until the end of March. Altitude was
suggested by Hughes (1987) to exert a large influence on the onset of flowering
with lower coastal sites flowering well before sites at higher altitudes (i.e.
up to 1,200 m). The periodicity of pod ripening is partly dependent upon the
climatic conditions and typically takes 45-60 days. Gliricidia sepium in
cultivation in wet areas may often flower, although sets little if any fruit.
Seeds are shed from pods through explosive
dehiscence with seed dispersal distances of up to 40 m (Simons and Dunsdon
1992). No scarification or pretreatment of seeds is required prior to
germination, and germination rates above 90% are typical. Following germination,
trees grow extremely quickly and may attain a height of 3 m before flowering at
age 6-8 months (Simons and Dunsdon 1992). Its rapid growth makes it an
aggressive pioneer capable of colonising secondary forest and fallow Imperata
dominated grassland often forming dense, pure stands (Anoka et al.
1991).
Individual trees display vast numbers of flowers
(up to 30,000) which attract a wide variety of insect visitors. Foremost amongst
these is a conspicuous species of carpenter bee (Xylocopa fimbriata)
that was suggested by Janzen (1983) and confirmed by Simons and Dunsdon
(1992) to be the primary pollinator of G. sepium. Xylocopa fimbriata is a
large (up to 30 mm in length), solitary bee that is principally attracted to the
abundant nectar of G. sepium, and is capable of flight distances of
several kilometres thus effecting pollen dispersal at great distances between
parents. Another genus of large bees (Centric sp.) was also
observed to visit G. sepium trees in Guanacaste, Costa Rica (Coville
et al. 1986).
The temperature requirements of G. sepium
are not too exacting as shown by the wide variation in mean monthly temperature
(20.7-29.2░C) at native sites. It will, however, not tolerate frosts which
partly explains its absence above 1,200 m in the native range. Whiteman et
al. (1986) in southeast Queensland, found that trees became leafless when
night temperatures fell below 15░C. Gliricidia can, however, be managed in a
coppice system in areas with light frost, by cutting the new growth before
frosts occur (Stewart et al. 1992).
 The 30 sites sampled by Hughes (1987) in his
range-wide collection of populations of G. sepium, represent a great
diversity of soil types. Most of the soils were highly eroded, of acid reaction
(pH 4.5-6.2) originating from volcanic parent material but also included sands,
heavy clays and calcareous limestone soils which were slightly alkaline. At
exotic locations, such as Peru, Szott et al. (1991) suggested that G.
sepium was suitable for acid, infertile soils. Furthermore, Whiteman et
al. (1986) considered G. sepium to be well adapted to low calcium
soils in Australia, although G. sepium was seen to have poor survival on
Indonesian soils with high aluminium saturation (Dierolf and Yost 1989). The 30 sites sampled by Hughes (1987) in his
range-wide collection of populations of G. sepium, represent a great
diversity of soil types. Most of the soils were highly eroded, of acid reaction
(pH 4.5-6.2) originating from volcanic parent material but also included sands,
heavy clays and calcareous limestone soils which were slightly alkaline. At
exotic locations, such as Peru, Szott et al. (1991) suggested that G.
sepium was suitable for acid, infertile soils. Furthermore, Whiteman et
al. (1986) considered G. sepium to be well adapted to low calcium
soils in Australia, although G. sepium was seen to have poor survival on
Indonesian soils with high aluminium saturation (Dierolf and Yost 1989).
A common feature of seasonally dry regions of
Central America and Mexico is the perennial fires which burn through fallow
agricultural land and secondary forest. Gliricidia sepium tolerates fires
well and trees quickly resprout with arrival of the rains. The increased
frequency of fires through deliberate burning may be responsible for the high
occurrence of G. sepium in secondary vegetation and agricultural fallows.
Holm et al. (1979) report G. sepium
as a severe weed in Jamaica, whereas Hughes and Styles (1984) consider G.
sepium to have only a slight weediness hazard.
Native range
Standley and Steyermark (1946) were the first to document the native
distribution of G. sepium and recorded its occurrence up to an altitude
of 1,600 m from Mexico through Central America to northern South America.
Acceptance of this distribution by later reviews include those of NAS (1980) and
Falvey (1982). Given Lavin's (1987) investigations, however, the higher
elevation specimens may have been Hybosema ehrenbergii.
Hughes (1987) was the first to distinguish
between native and naturalised distributions of G. sepium in his
comprehensive genecological survey of the native range. In his tentative
distribution map, Atlantic coastal populations and northern South American
populations were assigned as naturalised thus restricting native sites to only
the dry forests of the Pacific coast in Mexico and Central America. The sites
sampled by Hughes ranged in altitude from sea level to 1,100 m, and in annual
rainfall from 650 to 3,500 mm.
Exotic distribution
The earliest documented case of the use of G. sepium as an exotic is
provided by Wiersum and Dirdjosoemarto (1987) who cite the Spaniards as taking
it to the Philippines in the early 1600s. It has also been used for several
centuries in the Caribbean where again the Spanish introduced it to shade cocoa
(Ford 1987). Gliricidia sepium was introduced into Sri Lanka in the 1700s
to shade tea plantations, although the Sri Lankan material came from Trinidad
where it is not native. This introduction was purportedly from seed of just one
tree (Hughes 1987). Liyanage (1987) records the presence of both white (G.
maculata) and purple (G. sepium) flowered trees of
gliricidia in Sri Lanka indicating several later introductions may have ensued.
From Sri Lanka, it has spread out to India, Indonesia, Malaysia and Thailand.
Similar introductions occurred in West Africa and Uganda to provide shade trees
for plantation crops (Atta-Krah 1987, Tothill 1940).
Most exotic introductions are from unknown origin
and are likely to be narrowly based. This supposition is supported by the
findings of Bumatay et al. (1987) who found local seed sources from the
Philippines to be inferior to the new collections made by Hughes (1987). Local
landraces in Sri Lanka, Indonesia and Nigeria have also been shown to be
outperformed by populations collected by Hughes (Simons and Dunsdon 1992).
Certain problems have emerged as a result of
growing G. sepium in exotic environments. Foremost among these are pest
and pathogen considerations. A number of insect pests attack G. sepium in
the Caribbean including aphids, mealy bugs and scale (Ford 1987). In India,
Subramaniam (1977) and Devasahayam et al. (1987) reported predation of
G. sepium by a bud weevil and a hepialid (Sahyadrassus malabaricus),
respectively.
Agnihothrudu (1961) reported problems with a
foliar disease (Pellicularia filamentosa) of Paraserianthes
falcataria being pathogenic to G. sepium. In addition, a root fungus
attacked G. sepium in Trinidad although Ford (1987) did not consider this
to be serious. Two foliar diseases were recorded on G. sepium in Nigeria,
namely Colletotrichum gloeosporioides and Cercosporidium gliricidiasis
(LennÚ and Sumberg 1986). LennÚ (1992) attributes the lack of many diseases
on gliricidia to its tendency to be leafless for periods of the year thus
reducing the likelihood of epidemics (Section 6.2).
Other biological problems have also arisen when
G. sepium is used as an exotic. The lack of flowering at sites where no
distinct dry season exists (e.g. Kalimantan, Indonesia; Seibert 1987) is
undoubtedly climatically induced. Where flowering occurs but no fruit develop to
maturity, climate is also likely to be implicated; however, the lack of suitable
pollinators may also account for this. Pod set was reported by Sumberg (1985) to
be particularly low in Nigeria Furthermore, Akkaseng et al. (1986)
emphasised the importance of identification of suitable rhizobial strains for
G. sepium when used as an exotic.
Uses
Few non-industrial tree species embody the
concept of a multipurpose tree better than G. sepium. Throughout both its
native and exotic ranges it is used to supply tree products such as fuelwood,
construction poles, crop supports, green manure, fodder and bee forage. In
addition, it is used in living fences, to stabilise soils and prevent erosion,
to shade plantation crops, as an ornamental and in traditional medicine for
eczema. Generally, however, it is cultivated for a particular purpose and the
additional benefits are appreciated but not necessarily demanded, thus the
concept of one individual tree supplying all of the above products is illusory.
A review of the main uses of
G. sepium is given below.
Fuel wood
The easy coppicing nature of G. sepium contributes to its acceptability
as a source of fuelwood. Fuelwood is obtained in its native range through the
occasional lopping of branches or by completely coppicing trees to low levels
above ground. Smaller diameter wood is not prized as much as larger diameters
because of its lower specific gravity. Most wood of G. sepium that is
collected is for self-consumption.
Wood of gliricidia burns slowly thus producing
good embers, and gives off little smoke or sparks explaining its general
acceptability (CATIE 1986). It has a good heating value (19.8 MJ/kg) with an
average specific gravity of 0.5-0.6 (Withington et al. 1987).
Accumulation of woody biomass by trees of G.
sepium is very much dependent on climate and soils, management, planting
density, length of rotation and the provenance used. Salazar (1986) reports dry
wood yields of up to 6.3 t/ha/year from trees in Costa Rica, whereas Wiersum
(1982) quotes yields of 1520 m3/ha/year. In the Philippines, where
G. sepium is grown in woodlots on a three-year rotation to provide wood for
tobacco curing, yields of up to 23-40 m3/ha/year have been obtained (Wiersum
and Dirdjosoemarto 1987).
An International Provenance Trial Series of G.
sepium was set up by the Oxford Forestry Institute (OFI) in the mid-1980s.
In total, more than 100 trials were established throughout the tropics under one
of two management systems, namely (i) pure-plot plantations for wood production
and (ii) hedgerow system for leaf production. The results from these trials
indicated that there were marked differences between provenances with up to 500%
differences in biomass production at some sites (Simons and Dunsdon 1992). One
provenance from Guatemala, Retalhuleu, showed stable and superior production for
both leaf and wood production across a wide range of sites. Another provenance
from Guatemala, Monterrico, showed poor growth in terms of wood production yet
was outstanding for leaf production. Progeny trials have now been set up of some
superior provenances so that genetic parameters may be calculated with a view to
converting the trials into seedling seed orchards to satisfy the demand for seed
of this species.
Living fences
A distinct advantage of G. sepium is its ability to root from cuttings or
stakes with high attendant survival. Stakes up to 2 m in length and 10-15 cm
diameter can be placed directly in the ground, a point reflected by one of its
common names, 'quick stick'. The benefit of using long stakes is that they are
not grazed out and compete better with other vegetation relative to seedlings.
Liyanage and Jayasundera (1989), however, reported that plants of G. sepium
grown from seed were more productive, hardier and developed a deeper rooting
system than plants derived from cuttings.
Several thousands of kilometres of living fences
have been planted in both dry and wet sites throughout Central America and
Mexico. These are commonly pollarded at a height of 1.0-2.5 m, and generally at
least once per year. Individual posts may last beyond 30 years whilst loppings
provide a ready supply of replacement posts. Loppings may also be used for
animal forage or firewood whilst the spreading crowns of fenceline trees give
shade and shelter to livestock. Living fences are used in the native range by a
wide cross-section of the community from wealthy cattle ranchers who use it for
pasture fences to resource-poor campesinos who use it to mark boundaries and
keep livestock out of cropped fields. Homestead gardens or domestic livestock
may also be fenced off with closely spaced living fences of G. sepium.
At exotic locations, gliricidia has also been
used extensively as a living fence. In Bali, slanting interweaved cuttings of
close spacing are used to create wire-free fences (Figure 2.2.2), or
alternatively, larger cuttings are used to support bamboo poles strung between
them. Sri Lankans frequently use very closely spaced smaller diameter cuttings
to create a dense barrier around home gardens.
Considerable research has been carried out on the
appropriate age of cuttings, method of propagation, best length and diameter,
and even on the optimal lunar phase when cuttings should be taken (Duguma 1988,
Yamoah and Ay 1986, Withington et al. 1987).
Green manure
A less historic use of gliricidia but one that is increasing in occurrence is
the use of leaves as a green manure; however, only isolated examples of mulching
or incorporation of leaves into soil (e.g. El Gariton, Guatemala) are evident in
the native range. Greater use of gliricidia as a green manure has been made
outside the native range with reports as early as the 1930s in Malaysia (Anon.
1934) and Sri Lanka (Joachim and Kandiah 1934) on its benefits.
In Sri Lanka, gliricidia has been grown between
rows of coconuts and found to be an excellent organic fertiliser (Liyanage
1987). In Western Samoa, taro yields have been increased by up to 54% with the
addition of gliricidia leaf mulch (Kidd and Taogaga 1985). Leaf mulch of G.
sepium increased the yield and reduced time to harvest of yam tubers in the
Ivory Coast (Budelman 1989). Similarly, rice yields were boosted by up to 77%
through the use of G. sepium mulch (Gonzal and Raros 1988). In addition,
where G. sepium was used as a mulch in rice fields, the incidence of a
rice leaf blight disease was reduced through stimulating growth of saprophytes
parasitic to the causal organism (Rajan and Alexander 1988).
Patil (1989) stated that 1 tonne dry weight of
leaves was equivalent to 27 kg N while Kang and Mulongoy (1987) reported that up
to 15 t/ha/year of gliricidia leaf biomass could be produced on good soils in
Nigeria providing the equivalent of 40 kg N/ha/year. These figures are likely to
be underestimates since they do not account for nutrients arising from the
sloughing of roots and nodules after pruning. Bindumadhava Rao et al.
(1966) reported that 400 coppiced trees grown around the field perimeter could
provide sufficient fertiliser for 1 ha of paddy rice.
The half-life of prunings of G. sepium
reported by Wilson et al. (1986) to be 20 days, has been found to be
relatively short compared with that of Leucaena leucocephala and
Flemingia macrophylla (Budelman 1988).
The timing and frequency of coppicing to produce
the most biomass at the right time of year was investigated by Ella et al.
(1989) in Sulawesi, Indonesia. They found that the optimal cutting interval
of hedges of G. sepium was 12 weeks and that higher densities, even up to
40,000 trees per hectare, were preferable to lower densities. Widiarti and
Alrasjid (1987), also in Indonesia, concluded there was no difference in biomass
production from coppicing heights of 20, 40 or 60 cm above ground.
Shade
Gliricidia sepium derives many of its common names (e.g. madre de cacao)
from its use in its native range to shade cocoa and coffee plantations. As an
exotic, G. sepium has also been used extensively as a shade tree and the
largest single cocoa plantation in the world (12,000 ha), in Indonesia, uses
G. sepium as the sole shade tree (Seibert 1987). The landraces which have
developed in exotic locations are largely remnants of populations chosen for
their arboreal form and may not be optimally suited for other uses.
An additional benefit found from shading tea in
Sri Lanka with trees of G. sepium was reduction in the incidence of
termites (Kathiravetpillai 1990).
Use of
Gliricidia as a Forage
Gliricidia is an important forage crop in
cut-and-carry systems in many parts of the tropics including southeast Asia, Sri
Lanka and the Caribbean (Falvey 1982, Chadhokar 1982). In other areas such as
West Africa, India and the Philippines, however, its use is severely limited by
apparent palatability problems (Mahadevan 1956, Trung 1989). Gliricidia is also
little used as forage within its native range in Central America This is partly
because extensive grazing systems are preferred over stall feeding in Central
America but there may also be a palatability constraint since little grazing of
trees is evident. In Costa Rica, for example, prunings from live fences are
sometimes left outside the fields, out of reach of the cattle, even where the
pasture is in poor condition.
Despite these mixed perceptions of gliricidia as
a forage crop, its use has been widely promoted and researched, due largely to
its high productivity and quality. Interest in gliricidia for fodder has
increased in recent years following the widespread defoliation of Leucaena
leucocephala by the psyllid. Gliricidia is one of the few forage tree
species capable of leaf yields comparable to those of leucaena and it will grow
on a wider range of soils tolerating low pH provided that this is not associated
with high aluminium saturation.
Leaf biomass production
Gliricidia resprouts vigorously after lopping and will tolerate repeated
cutting. Moreover, its phenology is affected by cutting, with resprouts
retaining their leaves in the dry season in the tropics when older shoots are
deciduous. Management by lopping thus greatly enhances the value of gliricidia
as a dry season forage.
Numerous studies have measured leaf biomass (dry
matter) production under a range of climatic and edaphic conditions, and under
various management regimes differing with respect to variables such as
establishment methods (seedlings versus stakes of various sizes), plant spacing,
lopping height and lopping frequency. Values reported for gliricidia annual leaf
dry matter production generally range from about 2 t/ha/year (Wong and Sharudin
1986) to 20 t/ha/year (Sriskandarajah 1987).
Ella et al. (1989) found that as plant
spacing was reduced, yield per plant decreased owing to competition, but total
forage yield per unit area increased, as did the leaf:wood ratio. They also
obtained the highest leaf yields at a planting density of 4 trees/m2,
the highest density tested. In hedgerow plantings, however, intra-row spacing
seems to have little effect on overall yield, as lower individual tree
productivity is compensated for by higher plant density. Atta-Krah and Sumberg
(1987) recommended an intra-row spacing of 10 cm, but found only small
differences in productivity for spacings ranging from 4 cm to 50 cm. In the same
study, plants propagated from stakes were initially much more productive than
those grown from seed, but by the fifth harvest (one year after the first) the
difference was no longer significant.
The ease of propagation from stakes is a major
advantage of gliricidia, especially as trees managed for leaf production with
frequent cutting may not flower and thus set no seed. Furthermore, seed
production in gliricidia depends on a marked dry season. Large (up to 1 m long)
stakes are generally found to give the best establishment and subsequent growth
(e.g. Adejumo 1991).
The optimum frequency of lopping for leaf
production depends on the local climate; clearly trees can be lopped more
frequently in the wet than in the dry season. In general, total annual biomass
yield increases with less frequent cutting, but as this also increases the
wood:leaf ratio the effect of cutting interval on leaf yield is less pronounced
(Ivory 1990). For gliricidia grown in the humid tropics and used only for
forage, a cutting interval of 6-12 weeks is usually recommended. On a
subtropical site in Australia, however, Gutteridge and MacArthur (1988) obtained
higher leaf yields from one harvest per year than from three to six harvests.
Nutritive value, anti-nutritional factors and
palatability
Gliricidia sepium leaves have a high feeding value, with crude protein
comprising 20-30% of the dry matter, a crude fibre content of only about 15%,
and in vitro dry matter digestibility of 60-65% (G÷hl 1981, Adejumo and
Ademosun 1985). Panjaitan (1988) found that in Indonesia, gliricidia leaves had
higher crude protein content in the wet season than in the dry season. Perera
et al. (1991) reported high digestibility of gliricidia in the rumen
relative to other multipurpose tree forages. Moreover, the dry matter
digestibility was increased by the addition of energy sources such as cassava to
the diet (Ademosum et al. 1985). Conversely, the digestibility of low
quality feeds can be increased by the addition of legume leaves (Ivory 1990)
(Section 4.2).
The apparent high quality of gliricidia leaves,
combined with high and sustainable biomass production, should make gliricidia at
least as important a forage crop as leucaena, but its use is severely limited by
palatability problems, as well as by concern over possible toxicity.
The toxic effects of gliricidia are well known in
its native range in Central America, where the leaves or the ground bark, mixed
with cooked maize, are used traditionally as a rodenticide (Standley and
Steyermark 1946). This toxicity is thought to be due to the conversion by
bacteria of coumarin to dicoumerol, a haemorrhagic compound, during
fermentation. There have also been reports of toxicity and growth inhibition in
other monogastric animals including poultry (Raharjo et al 1987) and
rabbits (Cheeke and Raharjo 1987). There is little evidence, however, of toxic
effects on ruminants fed either fresh or wilted leaves and gliricidia is also
relatively low in tannins compared with other forage tree legumes such as
Calliandra calothyrsus. According to Lowry (1990), the only real constraint
to its feed value for ruminants lies in its palatability. Animals seem to refuse
gliricidia leaves on the basis of smell, often rejecting it without tasting it,
which suggests that the problem lies with volatile compounds released from the
leaf surface.
The apparent variation in the acceptability of
gliricidia to animals remains a major enigma. In some areas such as Colombia and
Sri Lanka, there appears to be no palatability problem and gliricidia is
therefore one of the most important dry season forages in these areas. In an
experiment in Guatemala, voluntary intake of gliricidia by lactating cows was
higher than either leucaena or Guazuma ulmifolia (Vargas et al.
1987). Elsewhere, however, gliricidia is perceived as completely unacceptable to
animals and is not used at all as forage despite its high nutritive value. In
feeding trials in Nigeria where a Panicum/gliricidia mix was offered, Ndama
cattle selected out the grass and left the gliricidia (J. Cobbina, personal
communication). A number of methods are used to increase its acceptability.
These include wilting, addition of molasses or salt, and accustomisation of the
animals by prolonged exposure and/or penning with adapted animals.
Wilting gliricidia leaves for 12-24 h before
feeding is found to increase intake markedly in many of the areas where
gliricidia is used as forage, and is therefore recommended wherever palatability
problems occur (e.g. Hawkins et al. 1990). The reason for this effect is
not known but if, as suggested above, acceptability is limited by volatile
compounds given off from the leaves, wilting presumably changes the composition
of these volatiles resulting in a more acceptable odour.
Differences in management do not, however, fully
explain the apparent differences in palatability. For instance, Perera (1992)
reported that in Sri Lanka gliricidia cannot be used as a live fence in goat
pastures because of browsing of stems and bark as well as leaves, whereas in
other areas, the animals will not even eat the leaves unless they are wilted. In
the Philippines, Perino (1979) found that gliricidia was seldom browsed by
either wild or domestic animals. Other possible reasons for the variation in
palatability in different parts of the world include climatic or edaphic effects
on leaf chemical composition, differences in behaviour or in rumen flora between
animals in different places (whether genetically or environmentally caused), or
genetic variation in the gliricidia itself. There is some anecdotal evidence to
support this last theory: according to Glander (1977), for instance, howler
monkeys foraging in Costa Rica feed selectively on only a few gliricidia trees
in a large population. In a 'cafeteria' trial in Nigeria using 30 provenances of
gliricidia, sheep showed clear preferences for some provenances over others (A.
Larbi, personal communication). The hypothesis that differences in acceptability
are genetically determined is currently being tested in a project based at the
OFI using a combination of analytical techniques and feeding trials. If
significant differences between provenances are found, palatability should be
included among the selection criteria in future genetic improvement of
gliricidia.
Use of gliricidia as a feed
Gliricidia is generally used as a high protein supplement to low quality basal
feeds such as grass, straw and other crop residues. Supplementation levels vary
but are usually in the range 20-40%. There are numerous reports of increases in
weight gain and milk production in both large and small ruminants when
gliricidia forage is used as a supplement. Nochebuena and O'Donovan (1986)
reported that for Tabasco sheep in Mexico, both intake and dry matter
digestibility increased when gliricidia was used as a supplement, up to 30% of
the diet, with grass hay. Chadhokar and Kantharaju (1980) found that gliricidia
supplementation levels up to 80% increased survival and growth of Bannur ewes
and lambs in Sri Lanka, and Van Eys et al. (1986), among others, have
demonstrated an increase in live weight gain for goats fed Napier grass
supplemented with gliricidia. For large ruminants, Chadhokar and Lecamwasam
(1982) and Premaratne (1990) reported increases in live weight gain for milking
cows and buffalo respectively on low protein diets supplemented with gliricidia,
although supplementation levels over 50% are reported to cause tainting of the
milk.
Carew (1983) has suggested that G. sepium
may also be used as a sole protein source for ruminants. Indeed, in Sri Lanka
during the dry season, gliricidia is commonly the sole feed of domestic goats (Perera
1992). Liyanage and Wijeratne (1987), however, found that with Sri Lankan
heifers, a gliricidia/Bracharia milliformis (grass) mixture
(1:1) gave greater live weight gain than gliricidia alone. Kabaija and Smith
(1989) concluded that G. sepium could also provide all livestock mineral
requirements if fed as sole feed, except for Cu and P which may need to be
supplemented. However, the use of pure gliricidia is unusual, even during the
dry season. According to Preston and Leng (1987), the growth rate of steers in
Colombia fed on King grass supplemented with gliricidia increased curvilinearly
with supplementation level, with the highest growth rate at about 30%
gliricidia. This result is in agreement with much of the research published to
date, that about 30% is the level at which the gliricidia protein is most
effectively used, in mixture with low quality basal feeds.
Conclusions
Gliricidia sepium is an extremely
versatile plant which can fulfil a number of roles in smallholder agricultural
production systems. It is considered by many to be the second most important
multipurpose tree legume after Leucaena leucocephala in the humid
tropics. It is a species of wide-ranging soil and climatic adaptations.
Consequently, it has been transported to most tropical countries and is now
pantropical in distribution.
However, its value and benefits are not
universally accepted as there is still debate concerning the quality of its
forage. Mackenzie (1986) suggested it may not be a really useful exotic in rural
communities in West Africa despite its abundance in the landscape. Hughes (1987)
suggested that one reason for its poor performance in some areas may be a result
of early exotic introductions coming from a very narrow genetic base.
Nevertheless, gliricidia is an extremely valuable
plant in tropical farming systems and recent provenance evaluations coordinated
by the OFI have highlighted superior genotypes. These and other evaluation
studies will produce material that will further improve biomass production and
extend the ecological range of the plant and also help to overcome some of the
perceived deficiencies within the currently used provenances.
References
Adejumo, J.O. (1991) Effect of length and girth
of vegetative planting material upon forage yield and quality of Gliricidia
sepium. Tropical Agriculture 68, 63-65.
Adejumo, J.O. and Ademosun, A.A. (1985) Effect of
plant age at harvest and of cutting time frequency and height on the dry matter
yield and nutritive value of Gliricidia sepium and Cajanus cajan.
Journal of Animal Production Research 5, 1-12.
Ademosum, A.A., Jansen, H.G. and van Houtert, V.
(1985) Goat management research at the University of Ife. In: Sumberg, J.E. and
Cassady, K. (eds), Sheep and Goats in Humid West Africa. ILCA, Addis
Ababa, Ethiopia, pp. 32-41.
Agnihothrudu, V. (1961) Diseases of shade trees
of Tea. Annual Report 1961, Tocklai Experimental Station, Assam.
Akkaseng, R., Whiteman, P.C. and Date, R.A.
(1986) Rhizobium requirements for two accessions of Gliricidia maculata.
Tropical Grasslands 20, 26-29.
Anoka, U.A., Akobundu, I.O. and Okonkwo, S.N.C.
(1991) Effects of Gliricidia sepium and Leucaena leucocephala on
growth and development of Imperata cylindrica. Agroforestry Systems 16,
1-12.
Anonymous (1934) Green manures. Straits
Settlements and Federal Malaya States, Department of Agriculture Leaflet No. 7.
Atta-Krah, A. (1987) Gliricidia - Report to IDRC
on Research Project 3-P-83-0058. ILCA,, Ibadan, Nigeria, 74 pp.
Atta-Krah, A.N. and Sumberg, J.E. (1987) Studies
with Gliricidia sepium for crop/livestock production systems in West
Africa In: Withington, D., Glover, N. and Brewbaker, J.L. (eds), Gliricidia
sepium (Jacq.) Walp.:) Management and Improvement.
Proceedings of a workshop at CATIE, Turrialba, Costa Rica. NFTA Special
Publication 87-01, pp. 3143.
Bindumadhava Rao, R.S., Krishnan, R.H.,
Theetharappan, T.S., Sankaranarayanan, R. and Venkatesan, G. (1966) A note on
gliricidia shrubs. Madras Agricultural Journal 60, 17-22.
Budelman, A. (1988) The decomposition of the leaf
mulches of Leucaena leucocephala, Gliricidia sepium and Flemingia
macrophylla under humid tropical conditions. Agroforestry Systems 7,
3345.
Budelman, A. (1989) Nutrient composition of the
leaf biomass of three selected woody leguminous species. Agroforestry Systems
8, 39-51.
Bumatay, E.C., Escalada, R.G. and Buante, C.R.
(1987) Preliminary study on the Gliricidia sepium germplasm collection in
Visca. In: Withington, D., Glover, N. and Brewbaker, J. (eds), Gliricidia sepium
(Jacq.) Walp.: Management and Improvement, Proceedings of a
workshop at CATIE, Turrialba, Costa Rica. NFTA Special Publication 87-01, pp.
162-167.
Carew, B.A.R. (1983) Gliricidia sepium as
sole feed for small ruminants. Tropical Grasslands 17, 181-184.
CATIE (1986) Silvicultura de especies promisorias
pare produccion de lena en America Central. Serie Tecnica. Informe Tecnico 86.
CATIE, Turrialba, Costa Rica. (In Spanish).
Chadhokar, P.A. (1982) Gliricidia maculata.
A promising legume forage plant. World Animal Review 44, 36-43.
Chadhokar, P.A. and Kantharaju, H.R. (1980)
Effect of Gliricidia maculata on growth and breeding of Bannur ewes.
Tropical Grasslands 14, 78-82.
Chadhokar, P.A. and Lecamwasam, A. (1982) Effect
of feeding Gliricidia maculata to milking cows a preliminary report.
Tropical Grasslands 16, 4648.
Cheeke, P.R. and Raharjo, Y.C. (1987) Evaluation
of Gliricidia sepium forage as leaf meal as feedstuffs for rabbits and
chickens. In: Withington, D., Glover, N. and Brewbaker, J.L. (eds), Gliricidia
sepium (Jacq.) Walp.: Management and Improvement. Proceedings of a
workshop at CATIE, Turrialba, Costa Rica. NFTA Special Publication 87-01, pp.
193-198.
Coville, R.E., Frankie, G.W., Buchmann, S.L.,
Vinson, S.B. and Williams, H.J. (1986) Nesting and male behavior of Centris
heithausi in Costa Rica with chemical analysis of the hindleg glands of
males. Journal of Kansas Entomological Society 59, 325-336.
Devasahayam, S., Premkumar, T. and Koya, K.M.A.
(1987) Record of Sahyadrassus malabaricus damaging Gliricidia maculata,
a standard of black pepper in Kerala. Entomon 12, 391-392.
Dierolf, T.S. and Yost, R.S. (1989) Survival
rates of three tree species in a four-year-old alley cropping trial. Nitrogen
Fixing Tree Research Reports 7, 12-13.
Duguma, B. (1988) Establishment of stakes of
Gliricidia sepium and Leucaena leucocephala. Nitrogen Fixing Tree
Research Reports 6, 6-9.
Ella, A., Jacobsen, C., StŘr, W.W. and Blair, G.
(1989) Effect of plant density and cutting frequency on the productivity of four
tree legumes. Tropical Grasslands 23, 28-34.
Falvey, J.L. (1982) Gliricidia maculate - a
review. International Tree Crops Journal 2, 1-14.
Ford, L.B. (1987) Experiences with Gliricidia
sepium in the Caribbean. In: Gliricidia sepium (Jacq.) Walp.:
Management and Improvement, Proceedings of a workshop at CATIE, Turialba,
Costa Rica. NFTA Special Publication 87-01, pp. 3-7.
Glander, K.E. (1977) Poison in a monkey's Garden
of Eden. Natural History (New York) 86, 34-41.
G÷hl, B. (1981) Tropical feeds; feed information
summaries and nutritive values. FAO Animal Production and Health Series, No. 12.
FAO, Rome, Italy, 529 pp.
Gonzal, D.G. and Raros, R.S. (1988) Effects of
Gliricidia sepium mulch on upland rice yield and soil fertility. In:
Multipurpose Tree Species for Small Farm Use. Proceedings of workshop,
Pattaya, Thailand. Winrock International, USA, pp. 261264.
Gutteridge, R.C. and MacArthur, S. (1988)
Productivity of Gliricidia sepium in a subtropical environment.
Tropical Agriculture 65, 275-276.
Hawkins, R., Sembiring, H., Lubis, D. and
Suwardjo, H. (1990) The potential of alley cropping in the uplands of East and
Central Java: A review. Upland Agriculture and Conservation Project, Farming
Systems Research, Agency for Agricultural Research and Development, Dept of
Agriculture, Indonesia, 71 pp.
Holm, L.G., Pancho, J.V., Herberger, J.P. and
Plucknett, D.L. (1979) A Geographical Atlas of World Weeds. Wiley, New
York, USA, 390 pp.
Hughes, C.E. (1987) Biological considerations in
designing a seed collection strategy for Gliricidia sepium. Commonwealth
Forestry Review 66, 31-48.
Hughes, C.E. and Styles, B.T. (1984) Exploration
and seed collection of multiple-purpose dry zone trees in Central America.
The International Tree Crops Journal 3, 1-31.
Ivory, D.A. (1990) Major characteristics,
agronomic features and nutritional value of shrubs and tree fodders. In:
Devendra, C. (ed.), Shrubs and Tree Fodders for Farm Animals. Proceedings
of a workshop in Denpasar, Indonesia, 24-29 July 1989, pp. 22-38.
Janzen, D.H. (1983) Costa Rican Natural
History. University of Chicago Press, Chicago, 816 pp.
Joachim, A.W.R. and Kandiah, P.R. (1934) The
change in composition and decomposability of typical Ceylon green manures with
age. Tropical Agriculturalist 82, 3-20.
Joseph, K., Menon, P.K.G. and Anilakumar, K.
(1991) Studies on the comparative efficiency of nitrogen sources in lowland
rice. Indian Journal of Agronomy 36, 122-123.
Kabaija, E. and Smith, O.B. (1989) Influence of
season and age of regrowth on the mineral profile of Gliricidia sepium
and Leucaena leucocephala. Tropical Agriculture 66, 125-128.
Kang, B.T. and Mulongoy, K. (1987) Gliricidia
sepium as a source of green manure in an alley cropping system. In:
Withington, D., Glover, N. and Brewbaker, J.L. (eds), Gliricidia sepium (Jacq.)
Walp.: Management and Improvement, Proceedings of a workshop at CATIE,
Turialba, Costa Rica. NFTA Special Publication 87-01, pp. 44-49.
Kathiravetpillai, A. (1990) The role of shade
trees in tea plantations. In: Gunasena, H.P.M. (ed.), Multipurpose Tree
Species in Sri Lanka. Proceedings of workshop, Kandy, Sri Lanka, pp. 37-44.
Kidd, T.J. and Taogaga (1985) Nitrogen fixing
trees as green manure for upland taro in Western Samoa. Nitrogen Fixing Tree
Research Reports 3, 67-68.
Lavin, M. (1987) A cladistic analysis of the
tribe Robineae. In: Stirton, C.H. (ed.), Advances in Legume Systematics,
Part 3. Royal Botanic Gardens, Kew, pp. 31-64.
Lavin, M., Matthews, S. and Hughes, C.E. (1991)
Chloroplast DNA variation in Gliricidia sepium (Leguminosae): Intra
specific phylogeny and tokogeny. American Journal of Botany 78,
1576-1585.
LennÚ, J.M. (1992) Diseases of multipurpose woody
legumes in the tropics: a review. Nitrogen Fixing Tree Research Reports
10, 13-32.
LennÚ, J.M. and Sumberg, J. (1986) Two foliar
diseases of Gliricidia sepium. Nitrogen Fixing Tree Research Reports 6,
31.
Liyanage, L.V.K. (1987) Traditional uses of
gliricidia in Sri Lanka. In: Withington, D., Glover, N. and Brewbaker, J.L. (eds),
Gliricidia sepium (Jacq.) Walp.: Management and Improvement.
Proceedings of a workshop at CATIE, Turrialba, Costa Rica. NFTA Special
Publication 87-01, pp. 92-94.
Liyanage, M. de S. and Jayasundera, H.P.S. (1989)
Effects of shading on seedling growth of Gliricidia sepium.) Nitrogen
Fixing Tree Research Reports 7, 95-96.
Liyanage, L.V.K. and Wijeratne, A.M.U. (1987)
Uses and management of Gliricidia sepium in coconut plantations of Sri
Lanka. In: Withington, D., Glover, N. and Brewbaker, J.L. (eds), Gliricidia
sepium (Jacq.) Walp.: Management and Improvement, Proceedings of a
workshop at CATIE, Turrialba, Costa Rica. NFTA Special Publication 87-01, pp.
95-101.
Lowry, J.B. (1990) Toxic factors and problems:
methods of alleviating them in animals. In: Devendra, C. (ed.), Shrubs and
Tree Fodders for Farm Animals. Proceedings of a workshop in Denpasar,
Indonesia, 24-29 July 1989, pp. 76-88.
Mackenzie, C.A. (1986) Report on a visit to the
West African evaluation network for Gliricidia sepium of ILCA. Oxford
Forestry Institute, 16 pp.
Mahadevan, V. (1956) Nutritive value of green
manure crops. 2. Gliricidia maculata. Indian Veterinary Journal 32, 457.
NAS (1980) Firewood Crops - Shrub and Tree
Species for Energy Production, National Academy Press, Washington DC.
Nochebuena, G. and O'Donovan, P.B. (1986) The
nutritional value of high-protein forage from Gliricidia sepium. World Animal
Review 57, 48-49.
Panjaitan, M. (1988) Regional evaluation of tree
legume species in Indonesia. M.Sc. thesis (Rural Science), University of New
England, Armidale, Australia, 138 pp.
Patil, B.P. (1989) Cut down fertilizer nitrogen
need of rice by Gliricidia green manure. Indian Farming 39, 34-35.
Perera, A.N.F. (1992) Gliricidia as a
fodder in livestock production - Sri Lankan experience. In: Multipurpose Tree
Species in Sri Lanka. Winrock International F/FRED, Peradeniya, Sri Lanka,
pp. 72-84.
Perera, A.N.F., Yaparatne, V.K. and van Bruchem,
J. (1991) Characterization of protein in some Sri Lankan tree fodders and
milling by products by nylon bag degradation studies. In: Proceedings of an
international workshop on Livestock and Feed Development in the Tropics, Malang,
East Java, Indonesia.
Perino, J.M. (1979) Rehabilitation of a denuded
watershed through the introduction of Kakawate (Gliricidia sepium).
Sylvatrop 4, 49-68.
Pertchik, B. and Pertchik, H. (1951) Flowering
Trees of the Caribbean. Rhinehart & Co., New York, 125 pp.
Premaratne, S. (1990) Effect of non-protein
nitrogen and fodder legumes on the intake, digestibility and growth parameters
of buffaloes. In: Domestic Buffalo Production in Asia. Proceedings of the
final research coordination meeting on the use of nuclear techniques to improve
domestic buffalo production in Asia - phase II, Rockhampton, Australia.
International Atomic Energy Agency, Vienna, Austria.
Preston, T.R. and Leng, R.A. (1987) Matching
Ruminant Production Systems with Available Resources in the Tropics and
Sub-tropics. Penambul Books, Armidale, Australia.
Raharjo, Y.C., Cheeke, P.R., Arscott, G.H.,
Burke, J.M. and Glover, N. (1987) Performance of broiler chicks fed
Gliricidia leaf meal. Nitrogen Fixing Tree Research Reports 5, 44-45.
Rajan, K.M. and Alexander, S. (1988) Management
of sheath blight disease of rice with Trichoderma viride and some soil
amendments in relation to the population of pathogen in soil. Journal of
Biological Control 2, 36-41.
Reynolds, S.G. (1988) Pastures and cattle under
coconuts. FAO Plant Production and Protection Paper, No. 91, 321 pp.
Rico-Gray, V., Chemas, A. and Mandujano, S.
(1991) Uses of tropical deciduous forest species by the Yucatan Maya.
Agroforestry Systems 14, 149-161.
Rydberg, P.A. (1924) Robinianae. North
American Flora 24, 220-249.
Salazar, R. (1986) Genetic variation in seeds and
seedlings of ten provenances of Gliricidia sepium. Forest Ecology and
Management 16, 391-401.
Seibert, B. (1987) Management of plantation cocoa
under gliricidia. In: Withington, D., Glover, N. and Brewbaker, J.L. (eds),
Gliricidia sepium (Jacq.) Walp.: Management and Improvement.
Proceedings of a workshop at CATIE, Turialba, Costa Rica. NFTA Special
Publication 87-01, pp. 102-110.
Simons, A.J. and Dunsdon, A.J. (1992) Evaluation
of the potential for genetic improvement of Gliricidia sepium. Report to
ODA on Forestry Research Project R.4525. Oxford Forestry Institute, 176 pp.
Sriskandarajah, N. (1987) Forage yield from
Gliricidia sepium in Papua New Guinea. Nitrogen Fixing Tree Research
Reports 5, 49-50.
Standley, P.C. and Steyermark, J.A. (1946) Flora
of Guatemala: Leguminosae. Fieldiana Botany 24 Part V, pp. 264-266.
Stewart J.L., Dunsdon, A.J., Hellin, J.J. and
Hughes, C.E. (1992) Wood Biomass Estimation of Central American Dry Zone
Species. Tropical Forestry Paper 26, Oxford Forestry Institute, 83 pp.
Subramaniam, T.R. (1977) Bionomics of the red
gram bud weevil. Journal of Entomological Research (New Dehli) 1, 40-46.
Sumberg, J.E. (1985) Note on flowering and seed
production in a young Gliricidia sepium seed orchard. Tropical
Agriculture 62, 17-19.
Szott, L.T., Palm, C.A. and Sanchez, P.A. (1991)
Agroforestry in acid soils of the humid tropics, Advances in Agronomy 45,
275-301.
Tothill, J.D. (1940) Agriculture in Uganda.
Oxford University Press, London, 435 pp.
Trung, L.T. (1989) Availability and use of shrubs
and tree fodders in the Philippines. In: Devendra, C. (ed.), Shrubs and Tree
Fodders for Farm Animals. Proceedings of a workshop in Denpasar, Indonesia,
pp. 279-294.
Van Eys, J.E., Mathius, I.W., Pongsapan, P. and
Johnson, W.L. (1986) Foliage of the tree legumes Gliricidia, Leucaena and
Sesbania as supplement to Napier grass diets for growing goats.
Journal of Agricultural Science 107, 227-234.
Vargas, B., Hugo, E., Pablo, G. and Elvira S.
(1987) Composicion quimica, digestibilidad y consumo de leucaena (Leucaena
leucocephala), madre de cacao (Gliricidia sp.) y
caulote (Guazuma ulmifolia). In: Withington, D., Glover, N. and
Brewbaker, J.L. (eds), Gliricidia sepium (Jacq.) Walp.: Management and
Improvement. Proceedings of a workshop at CATIE, Turrialba Costa Rica. NFTA
Special Publication 87-01, pp. 217-222. (In Spanish.)
Whiteman, P.C., Oka G.M., Marmim, S., Chand, S.
and Gutteridge, R.C. (1986) Studies on the germination, growth and winter
survival of Gliricidia maculata in southeastern Queensland.
International Tree Crops Journal 3, 245-255.
Widiarti, A. and Alrasjid, H. (1987) Introduction
of fuelwood tree species on degraded lands in Paseh and Kadipaten areas in
Indonesia. Bulletin Penelitian Hutan 10, 1-17.
Wiersum, F. (1982) Fuelwood as a traditional and
modern energy source in the Philippines. Working Paper No. 6. FAO, Manila.
Wiersum, F. and Dirdjosoemarto, S. (1987) Past
and current research with gliricidia in Asia. In: Withington, D., Glover, N. and
Brewbaker, J.L. (eds), Gliricidia sepium (Jacq.) Walp.: Management and
Improvement. Proceedings of a workshop at CATIE, Turrialba, Costa Rica NFTA
Special Publication 87-01, pp. 20-28.
Wilson, G.F., Kang, B.T. and Mulongoy, K. (1986)
Alley cropping: trees as sources of green manure and mulch in the tropics.
Biological Agriculture and Horticulture 3, 251-267.
Withington, D., Glover, N. and Brewbaker, J.L. (eds),
(1987) Gliricidia sepium (Jacq.) Walp.: Management and Improvement.
Proceedings of a workshop at CATIE, Turrialba, Costa Rica. NFTA Special
Publication 87-01, 255 pp.
Wong, C.C. and Sharudin, M.A.M. (1986) Forage
productivity of three forage shrubs in Malaysia. MARDI Research Bulletin
14, 178-188.
Yamoah, C.F. and Ay, P. (1986) The effects of
some methods of establishing Gliricidia sepium on food crop performance,
growth and survival rate of Gliricidia. International Tree Crops Journal
4, 17-31.
.Compiled by Muhammad
Irfan
Curtsey: FAO & Dr A. A. Quraishy
|
Pakissan.com;
|